Titanium: Types, Characteristics, and Properties
- Product
- titanium

※)The content of this article reflects our opinion and does not guarantee completeness or accuracy. When purchasing or processing products, please consult with specialized professionals, including our company, and verify the material based on its intended purpose and application before use.
What kind of image do you have of titanium? In everyday items, perhaps something like this: “Eyeglass frames, wristwatches, driver heads, bicycles, water bottles, etc. They’re expensive, but lightweight and rust-resistant.
Compared to metals like iron, titanium has a relatively short history, and its application range is somewhat limited. However, as a new material, improvements are being made, and its uses are expanding. I would like to take this opportunity to reintroduce titanium.
The crystal structure of titanium
Pure titanium (Ti) exhibits a hexagonal close-packed (hcp) crystal structure at room temperature, which is referred to as the α-phase. When heated to high temperatures, it undergoes an allotropic transformation at 885°C, changing to a body-centered cubic (bcc) structure, known as the β-phase.
At room temperature, iron exhibits a body-centered cubic (bcc) structure, which is referred to as the α-phase. At higher temperatures, it transforms into a dense face-centered cubic (fcc) structure, known as the γ-phase. Care must be taken not to confuse these with the phases of titanium.
By adding alloys such as vanadium (V) to titanium (Ti), it is possible to achieve a state where the α-phase and β-phase coexist at room temperature, or a state where only the β-phase exists. Furthermore, through heat treatment, it is possible to achieve α+β phases or the β-phase, which typically occur at high temperatures, at room temperature. These phases significantly influence the material’s properties.
We started with a somewhat complex discussion, but the key points to understand here are that titanium phases are classified based on differences in crystal structure, which in turn affect performance, and that titanium materials can be broadly divided into two categories: pure titanium and titanium alloys.
Types and Characteristics of Titanium
Pure Titanium Category
When it comes to titanium as a material, the first thing that comes to mind is something close to pure titanium.
Titanium has a relatively low specific gravity of 4.51, making it approximately 60% the weight of iron (7.87) or stainless steel. Although it is not as light as aluminum, it is still perceived as a lightweight material. Additionally, while stainless steel has better corrosion resistance compared to ordinary iron (steel), titanium outperforms stainless steel even in environments such as seawater, which stainless steel struggles with. It can be said that titanium is an exceptionally corrosion-resistant material.
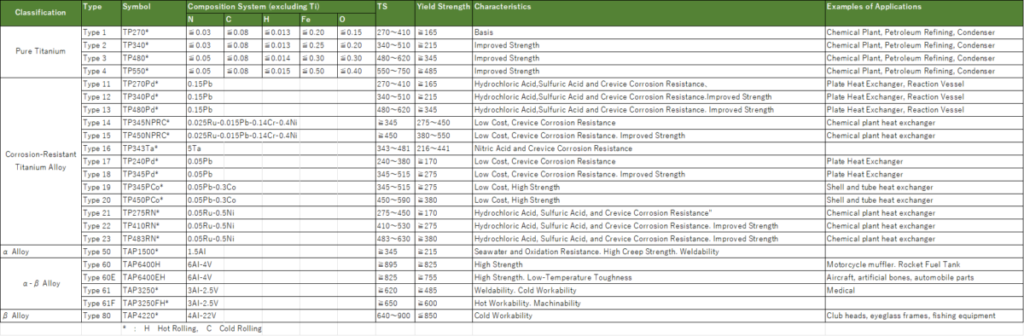
As shown in Table 1, the Japanese Industrial Standard (JIS), specifically JIS H4600, categorizes titanium into Types 1 through 4. The higher the type number, the greater the content of Fe (iron) and O (oxygen), resulting in higher strength.
It covers a strength range from mild steel, commonly used in general steel sheets, to approximately 60 kg steel. Due to its low specific gravity, its strength-to-weight ratio (specific strength) is relatively high. The most commonly used types are Type 1 and Type 2, which have relatively lower strength.
Alloy Titanium Category
In addition to pure titanium, corrosion-resistant titanium alloys are created by adding trace elements to further enhance corrosion resistance.
In JIS H4600, Types 11 to 13 are defined as titanium alloys created by adding approximately 0.15% palladium (Pd) to Types 1 to 3. This addition helps suppress corrosion caused by hydrochloric acid and sulfuric acid while improving crevice corrosion resistance. Additionally, Types 14 to 23 are designated as corrosion-resistant titanium alloys with elements such as ruthenium (Ru), tantalum (Ta), cobalt (Co), chromium (Cr), and nickel (Ni) added. These alloys are used as required, including versions designed to lower costs by reducing the amount of expensive Pd, or versions aimed at suppressing corrosion from nitric acid.
Manufacturers sell titanium designed for specialized applications not specified in JIS standards under their own unique brand names.
These corrosion-resistant properties are limited in application and are therefore not versatile. Additionally, what are referred to as corrosion-resistant titanium alloys basically fall under the category of α-alloys.
Furthermore, there are alloy-based titanium materials where the metallic phases are controlled. Titanium alloys are classified into three types: α-alloys, α-β alloys, and β-alloys. The most famous among them is the α-β alloy Ti-6%Al-4%V, which is categorized into 60 types under JIS standards. It features a mixed structure of both α-phase and β-phase.
It has been used in aircraft, particularly military aircraft, and has supported the development of titanium materials. Aluminum (Al) stabilizes the α-phase, but excessive addition can lead to the precipitation of Ti3Al, causing issues such as reduced toughness. Therefore, the addition is limited to 6% or less.

This titanium alloy combines the characteristic of high strength and heat resistance provided by aluminum (Al) addition, with the benefits of high toughness and workability achieved by adding vanadium (V), which stabilizes the β-phase. It is a well-balanced titanium alloy, and when people referred to titanium, they were often specifically referring to this titanium alloy.
As an α-alloy, there is the JIS Type 50, which includes 1.5% aluminum (Al). It exhibits high strength at elevated temperatures, excellent oxidation resistance at high temperatures, and workability comparable to pure titanium. It is used in applications such as motorcycle exhaust systems.
In the category of β-alloys, there is Ti-4%Al-22%V, classified under JIS as Type 80, which contains a significant amount of vanadium (22%). A notable feature of this alloy is that it can be formed and processed at room temperature when in a solution-treated state. After processing, its strength can be further enhanced through aging treatment.
Titanium is highly resistant to corrosion and does not leach, which minimizes its impact on metal allergies and makes it a highly biocompatible metal. Although vanadium (V), added as an alloy element, has not been observed to cause harm in small amounts, it is a toxic metal on its own, leading to the perspective that it is better avoided when possible.
Table 1 lists the standards for plates, but there are also standards for pipes, rods, and wires.
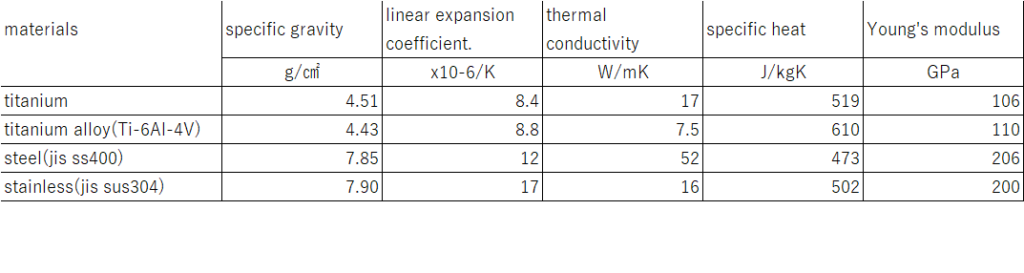
Apart from being lightweight and having excellent corrosion resistance, titanium has other characteristics as shown in Table 2.
A low coefficient of linear expansion means that the material does not expand easily even when the temperature rises, resulting in minimal deformation. Furthermore, its value is close to that of materials such as concrete, glass, and carbon fiber reinforced polymer (CFRP) used in aircraft, making it highly compatible with these materials.
A low thermal conductivity means that heat is not easily transferred, allowing it to be retained and maintained, which makes the material suitable for use as insulation. It is effective in items such as cups and pots. On the other hand, during machining, heat can accumulate, causing the temperature to rise, which leads to difficulties in processing.
A low Young’s modulus means that the material is prone to bending, which can lead to large displacements when force is applied, making it not necessarily ideal for use in structural applications. On the other hand, it offers advantages such as improving ride comfort by absorbing vibrations in bicycle frames, and the ability to easily return to its original shape even after deformation in eyeglass frames.
It is also non-magnetic, meaning it does not stick to magnets. While magnets cannot be used for fixation during processing, its non-magnetic property allows for various applications. For example, it is used in MRI peripheral equipment and electronic devices where it is important to avoid the influence of magnetism.
It is necessary to select materials while taking such characteristics of titanium into consideration.
In this column, we have explained the types, characteristics, and properties of titanium as a material. Moving forward, we will continue to provide information on specific applications of titanium. Please look forward to it!
“Our company is…”
We are a specialized trading company based in Japan, dealing in high-tensile steel and other special steels.
Please feel free to contact us.
Feel free to email us.
↓↓↓
info@kumagai-steel.co.jp

